Quality Is the key to unlocking billions of dollars in product value
Microsoft Dynamics 365 for Life Sciences
Bring safe, compliant and effective products to the Life Sciences market... faster.
Introduction
The biotechnology, pharmaceutical, and medical device industries are famously complex, with product owners and contract development and manufacturing organizations (CDMOs) performing different roles across the value chain. However, these parties benefit significantly when they improve quality incident data transparency, automate key processes, and use insights to improve decision-making across the enterprise.
Product owners have billions of dollars in development costs and future revenues riding on the successful launch of each new pharmaceutical drug, therapy, or medical device. Thus, it’s no surprise that quality executives at these companies seek to avoid costly incidents that can result in such issues as delays taking products to market, product recalls, and regulatory fines.
Finance and technology executives at these companies are accountable for their company’s quality and compliance processes and reputation, which can add or subtract hundreds of millions of dollars from their company’s market value. So, these leaders care about increasing quality incident data visibility, driving product volumes and operational velocity, and strengthening financial margins.
CDMOs may have a different focus. These organizations manage clinical trials, track vendor and manufacturer certification, oversee production processes, and distribute items directly or via third-party logistics. For leaders at these organizations, the biggest driver is time. With visibility into quality incident data and tighter control over related processes, CDMOs can speed up the time between trials and taking products to market. By so doing, they can increase their customers’ revenues and use these successes to win significant new business.
Quality Is the key to unlocking billions of dollars in product value
10.5 Years
Average time for an innovative new drug to progress through a Phase One program to regulatory approval.
$985M
Median capitalized R&D investment made by biopharmaceutical companies to take a new drug to market.
$526.4M
Average capitalized development investment made by manufacturing companies to develop a complex medical device.
The Many Benefits of Improving Quality Incident Data Transparency
Quality issues can occur at any time and across any point of the therapy, drug, or device lifecycle, from raw material sourcing and storage to manufacturing, logistics, and consumer usage. Incidents can range from simple infractions, such as not accurately measuring the gross weight of raw materials upon reception, to more damaging and costly incidents, such as manufacturing contaminated products or discovering counterfeit drug distribution. Quality incident data includes consumer complaints, non-conformances, incidents and related investigations, and their related corrective and preventive actions (CAPA).
Whether your company is a product owner or a CDMO, you are required by the U.S. Food and Drug Administration (FDA), MHRA in the UK, to track quality incident data through the CAPA process. Your quality teams must investigate incidents to identify root causes. These experts also establish corrective and preventive measures, where needed, to prevent their recurrence. Your quality teams may control this data tightly due to fears of unauthorized exposure and resulting regulatory risks.
However, there’s a different reality possible — one where your quality teams securely share incident data and collaborate on CAPA processes in real time. Your teams, ranging from supply chain and production to engineering and logistics, can then access this data, contribute to investigations, and share insights internally and with key partners. You’re able to turn CAPA processes into a well-oiled machine, reducing the cost to resolve incidents and decreasing their market impacts. As a result of improved quality processes and controls, your firm can accelerate speed to market for new products and safely increase capacity, unlocking new revenue growth.
Benefits of Sharing Quality Incident Data Internally
Anticipating and correcting conditions that could lead to quality incidents, preventing business and operational disruption.
Gaining the insights you need to address supply chain issues and drive operational output.
Negotiating better deals with suppliers, using quality data to drive preferential terms or defend a move to a new vendor.
Preventing unnecessary audits by providing the right data to internal teams upon request.
Planning proactive or preventive equipment
maintenance to avoid costly downtime or quality issues. Alternatively, a lack of issues might mean that you could reduce maintenance, saving on operational costs.
Positioning your firm for future equity investment by providing seamless access to digital quality data and processes. For early-stage companies, this might mean creating a faster path to IPO or winning more attractive terms during an M&A courtship. For more mature companies, it may mean increasing firm
revenues and profitability.
Overcoming the Challenges of Legacy Systems and Processes
So, it’s clear your company can use quality data to drive business value and operational improvement. Yet, you may struggle to address different challenges that mire you in the status quo. Your company uses analytics and technology to develop innovative drugs and biologics. So, why is quality incident data so difficult to centralize and share? There are multiple reasons:
Quality teams may be afraid to share data: Incident data that is accidentally or deliberately leaked outside your organization could lead to internal audits, regulatory findings, fines, or even jail time if the FDA or MHRA determines quality incidents have been mismanaged. As a result, your quality function may not want to release control over data. The irony is that digital solutions actually make it easier to control data than paper-based or low-tech processes, as they institute secure role-based controls. Sending PDFs and spreadsheets between quality reviewers is an inherently insecure process, as data can easily get diverted, lost, and misused.
Current processes work but may inhibit growth: Quality teams at early-stage organizations may view paper as the right way to handle processes, to minimize technical debt when funding is limited and focused on product development. As these organizations move to pre-clinical or pre-commercial operations, they must then meet FDA requirements. At this point, implementing proven software that optimizes quality processes and provides a real-time audit trail may demonstrate commercial readiness, allowing your firm to acquire significant private equity investment. You can demonstrate your ability to track and integrate all quality-related processes, from compliance, material development, and release, to CAPA processes and auditing, instilling confidence in your investors.
Reporting processes may be too flexible: Your company must meet stringent FDA reporting requirements. However, you have flexibility in how you gather, track, and manage quality data. As a result, your quality team may capture and store data on paper forms a variety of ways, including paper, emails, online data and documents, team sites, and in systems of record. However, manual processes don’t scale, and data usually gets trapped in silos. Even software that technically supports audits may provide records as a data dump, rather than organizing them in a logical and meaningful way. As a result, it’s difficult to standardize and enforce critical processes or share key learnings across your organization.
Quality incident data may be kept in three-ring binders, emails, Google Documents, Microsoft Excel spreadsheets, Microsoft SharePoint Sites, and other sources. In addition, auditable information may also be stored in laboratory information management systems (LIMS); quality management systems (QMS); and customer relationship management (CRM), enterprise resource planning (ERP), and supply chain management (SCM) systems of record.
Mergers and acquisitions tangle processes: In 2021, the healthcare and biopharmaceutical industry notched $288.9 billion in new deals, a 38-percent increase from the year before. Health industries M&A is set to rebound in 2023, with investors attracted by innovation and growth prospects. Constant mergers and acquisitions (M&As) can create an overabundance of legacy systems and processes that then have to be rationalized or retired. Merging quality incident processes across two or more acquired firms can be painful and long process.
The Five Steps That Lead to Quality Incident Data Sharing Enlightenment
As a quality, finance, or technology leader, you understand the opportunities for centralizing and sharing quality incident data. Here are five steps to help you accomplish that goal.
1. Develop a solid business case
Typically, a C-level leader, such as a chief financial, information, or quality officer (CFO, CIOO, CQO) will spearhead the drive to centralize quality incident data. Compliance leaders are also critical stakeholders, given this data’s criticality to meeting quality regulations such as 21 CFR 820 Quality System Regulation.
Your leaders may be driven by different motivations, such as standardizing and controlling CAPA processes with automation, leveraging their ERP platform to enable secure data sharing, or using quality incident data to drive better business outcomes. To bring other stakeholders on board, you will need to cast a vision for what centralizing data access can accomplish for other business units, how data will be secured in the ERP platform, and how controls and workflow will simplify operational processes.
Our team can work with your team to align around a shared vision, demonstrate the future state of quality incidents and the resulting CAPA processes when automated, and share success stories. As just one example, we worked with a leading global immunotherapy provider to implement the Merit for Life Science platform to meet advanced quality and compliance management requirements. The platform layers industry-specific functionality on top of Microsoft Dynamics 365 and is delivered via the Microsoft cloud. The immunotherapy provider used insights from Merit for Life Science to improve manufacturing, supply chain, inventory, and warehousing
processes.
2. Digitize existing processes
You already have solid processes to identify and address quality incidents. Thus, the goal is not to rearchitect them, but instead to analyze them for gaps or redundancies. By so doing, you can re-envision processes as automated sequences that reduce manual intervention, delays, and error.
Our team leverages a standard methodology, proven across many life sciences engagements, to guide you through this process. We identify key process owners and work with them through a solutioning phase.
We offer Merit for Life Science to meet biotechnology, pharmaceutical, and medical device companies’ unique business and operational requirements. This end-to-end ERP solution provides the quality, compliance, supply chain, planning, resourcing, manufacturing execution, and materials management functionality you need to grow your business in a validated, compliant manner.
We present default, out-of-the box processes and discuss their fit against your business needs. We then tailor functionality to your requirements and execute user testing to ensure their acceptance.
3. Share quality incident data with internal and external stakeholders
Now that you have cloud-enabled data, you can share it securely with other key stakeholders. Our team provides training on how to access data and use reporting to optimize key processes. Here are some of the ways quality incident data helps other business stakeholders:
In addition, centralizing quality data access is a key step on the path to creating a smart quality function, which can be a significant driver of business value. Biotechnology, pharmaceutical, and medical device companies that implement smart quality processes gain a sense-and-respond capability. That capability enables these companies to quickly identify emerging market trends and customer needs, align product investments to these trends, and turn compliance processes into a source of competitive advantage. In addition, they motivate employees and partners to become smart quality advocates, identifying issues and proposing changes that continuously bolster quality over time.
Further, as industry companies develop closer relationships with providers and patients, they have the opportunity to identify potential product quality and usage issues, collect data more rapidly, and respond to incidents proactively. By so doing, biotechnology, pharmaceutical, and medical device companies can integrate their quality incident management processes into an end-to-end plan-produce-deliver value chain. These companies enhance the customer experience by being responsive and provider- and patient-focused. They also reduce costs, such as widespread product recalls, that harm brand reputation, profitability, and market position.
Biotechnology, pharmaceutical, and medical device companies can use quality data to improve process efficiency and business effectiveness, outcompeting in their heavily regulated industries.
4. Benefit from secure data sharing processes
Users are assigned security roles with predefined access privileges, which your company can tailor to its needs. These roles use the concept of least privilege granted, providing only the data and tool access that users need to do their jobs. External partners can also be granted access to enter their data securely and directly into the Merit for Life Science platform, providing your organization with a real-time lens into quality performance.
In addition, quality and compliance teams can easily access audit trails for processes ranging from product testing to inventory management and lot release. With holistic visibility and consistent workflow, quality teams are able to identify incidents and work through CAPA processes swiftly and effectively with other stakeholders. This data also helps these teams defend decisions to regulators.
Biotechnology, pharmaceutical, and medical device companies benefit from embedding quality incident data and processes in the Microsoft cloud platform (also known as Microsoft Azure). As one of the world’s leading cloud providers, Microsoft uses a zero trust security model, continually validating user identities, devices, sessions, locations, and more for every access request.
Zero trust takes a multi-layered approach to security, using multiple solutions to enable advanced threat protection across the hybrid cloud. As just one example, Microsoft Azure responds to 24 trillion security signals per day. Microsoft also has more than 90 compliance certifications to meet regulatory requirements across industries and geographies.
With Microsoft’s industrial-grade ERP controls and cloud security, biotechnology, pharmaceutical, and medical device companies can rest assured that their quality incident data is protected from accidental or malicious exposure.
5. Commit to continuous improvement
Now that you have 24/7 visibility into quality incidents, you can partner with other business stakeholders on quality data initiatives. You can also use analytics to optimize processes, ranging from supplier management to employee onboarding and training and equipment maintenance.
The following dashboard image shows how we can enable key stakeholders to use quality data and analytics to make better decisions across the product lifecycle:
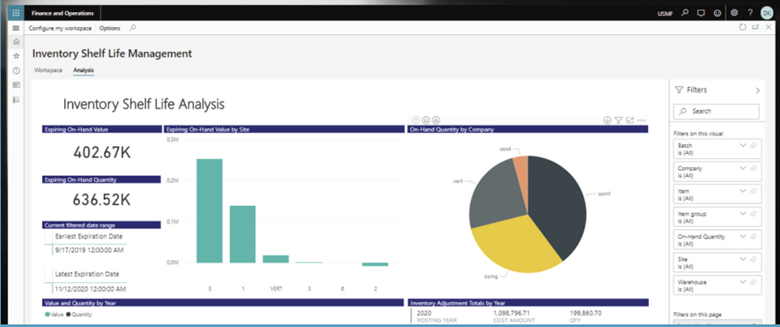
Why it’s Time to Digitize and Share Quality Incident Data
Your quality function helps guide your organization through regulatory compliance processes and safeguard your products, processes, and reputation. But your team is capable of so much more. Quality incident data and processes can be a driver of significant business value if shared with other stakeholders.
The path forward is to develop a shared vision and business case, digitize processes, share data securely with key stakeholders, and commit to continuous improvement. By doing so, your company can become a true quality leader. You’ll take more products to market, drive greater revenues and profitability, ensure seamless regulatory compliance, and attract the investment that will take your firm to the next level.
Microsoft Dynamics 365 for Life Sciences
Bring safe, compliant and effective products to the Life Sciences market... faster.
Want to know more about HSO's solutions and capabilities for the Life Science industry?
We, and third parties, use cookies on our website. We use cookies to keep statistics, to save your preferences, but also for marketing purposes (for example, tailoring advertisements). By clicking on 'Settings' you can read more about our cookies and adjust your preferences. By clicking 'Accept all', you agree to the use of all cookies as described in our privacy and cookie policy.
Purpose
This cookie is used to store your preferences regarding cookies. The history is stored in your local storage.
Cookies
Location of Processing
European Union
Technologies Used
Cookies
Expiration date
1 year
Why required?
Required web technologies and cookies make our website technically accessible to and usable for you. This applies to essential base functionalities such as navigation on the website, correct display in your internet browser or requesting your consent. Without these web technologies and cookies our website does not work.
Purpose
These cookies are stored to keep you logged into the website.
Cookies
Location of Processing
European Union
Technologies Used
Cookies
Expiration date
1 year
Why required?
Required web technologies and cookies make our website technically accessible to and usable for you. This applies to essential base functionalities such as navigation on the website, correct display in your internet browser or requesting your consent. Without these web technologies and cookies our website does not work.
Purpose
This cookie is used to submit forms to us in a safe way.
Cookies
Location of Processing
European Union
Technologies Used
Cookies
Expiration date
1 year
Why required?
Required web technologies and cookies make our website technically accessible to and usable for you. This applies to essential base functionalities such as navigation on the website, correct display in your internet browser or requesting your consent. Without these web technologies and cookies our website does not work.
Purpose
This service provided by Google is used to load specific tags (or trackers) based on your preferences and location.
Why required?
This web technology enables us to insert tags based on your preferences. It is required but adheres to your settings and will not load any tags if you do not consent to them.
Purpose
This cookie is used to store your preferences regarding language.
Cookies
Why required?
We use your browser language to determine which language to show on our website. When you change the default language, this cookie makes sure your language preference is persistent.
Purpose
This service is used to track anonymized analytics on the HSO.com application. We find it very important that your privacy is protected. Therefore, all data is collected and stored on servers owned by HSO with no third-party dependencies. This cookie helps us collect data from HSO.com so that we can improve the website. Examples of this are: it allows us to track engagement by page, measuring various events like scroll-depth, time on page and clicks.
Cookie
Purpose
This cookie enables HSO to run A/B tests across the HSO.com application. A/B testing (also called split testing) is comparing two versions of a web page to learn how we can improve your experience. All data is collected and stored on servers owned by HSO with no third-party dependencies.
Purpose
With your consent, this website will load Google Analytics to track behavior across the site.
Cookies
Purpose
With your consent, this website will load the Microsoft Clarity script, which helps us understand how people use the site. The cookies set by Clarity collect session-level data like how the visitor landed on the site, which pages they viewed, their language preference, and even their general location. This data powers Clarity’s features like heatmaps and session recordings, helping us see which parts of a page get attention and where users drop off. The goal isn’t to track individuals, but to understand patterns that can improve the user experience. Learn more about Microsoft Clarity cookies here.
Cookies
Technologies Used
Cookies
Purpose
With your consent, this website will load the Google Advertising tag which enables HSO to report user activity from HSO.com to Google. This enables HSO to track conversions and create remarketing lists based on user activity on HSO.com.
Possible cookies
Please refer to the below page for an updated view of all possible cookies that the Google Ads tag may set.
Cookie information for Google's ad products (safety.google)
Technologies Used
Cookies
Purpose
With your consent, we use IPGeoLocation to retrieve a country code based on your IP address. We use this service to be able to trigger the right web technologies for the right people.
Purpose
With your consent, we use Leadfeeder to identify companies by their IP-addresses. Leadfeeder automatically filters out all users visiting from residential IP addresses and ISPs. All visit data is aggregated on the company level.
Cookies
Purpose
With your consent, this website will load the LinkedIn Insights tag which enables us to see analytical data on website performance, allows us to build audiences, and use retargeting as an advertising technique. Learn more about LinkedIn cookies here.
Cookies
Purpose
With your consent, this website will load the Microsoft Advertising Universal Event Tracking tag which enables HSO to report user activity from HSO.com to Microsoft Advertising. HSO can then create conversion goals to specify which subset of user actions on the website qualify to be counted as conversions. Similarly, HSO can create remarketing lists based on user activity on HSO.com and Microsoft Advertising matches the list definitions with UET logged user activity to put users into those lists.
Cookies
Technologies Used
Cookies
Purpose
With your consent, this website will load the Microsoft Dynamics 365 Marketing tag which enables HSO to score leads based on your level of interaction with the website. The cookie contains no personal information, but does uniquely identify a specific browser on a specific machine. Learn more about Microsoft Dynamics 365 Marketing cookies here.
Cookies
Technologies Used
Cookies
Purpose
With your consent, we use Spotler to measures more extensive recurring website visits based on IP address and draw up a profile of a visitor.
Cookies
Purpose
With your consent, this website will show videos embedded from Vimeo.
Technologies Used
Cookies
Purpose
With your consent, this website will show videos embedded from Youtube.
Cookies
Technologies Used
Cookies
Purpose
With your consent, this website will load the Meta-pixel tag which enables us to see analytical data on website performance, allows us to build audiences, and use retargeting as an advertising technique through platforms owned by Meta, like Facebook and Instagram. Learn more about Facebook cookies here. You can adjust how ads work for you on Facebook here.
Cookies
Purpose
With your consent, we use LeadInfo to identify companies by their IP-addresses. LeadInfo automatically filters out all users visiting from residential IP addresses and ISPs. These cookies are not shared with third parties under any circumstances.
Cookies
Purpose
With your consent, we use TechTarget to identify companies by their IP address(es).
Cookies
Purpose
This enables HSO to personalize pages across the HSO.com application. Personalization helps us to tailor the website to your specific needs, aiming to improve your experience on HSO.com. All data is collected and stored on servers owned by HSO with no third-party dependencies.
Purpose
With your consent, we use ZoomInfo to identify companies by their IP addresses. The data collected helps us understand which companies are visiting our website, enabling us to target sales and marketing efforts more effectively.
Cookies